ABSTRACT
Theranostics, a combination of therapeutics and diagnostics, spans a spectrum of research areas to provide new opportunities in developing new healthcare technologies and medicine at affordable prices. Through employing a personalized medicine approach, biotechnology can be tailored to the needs of an individual. Applications of theranostics include drug delivery carriers capable of sustained drug release and targeted delivery, biosensors with high sensitivity and selectivity, and diagnostic relevant entities that can be incorporated into the former technologies.
Nanotechnology provides a suitable foundation for theranostics to build upon due to material-based properties; magnetism, biocompatibility, and quantum effects to name a few. Purpose can be incorporated and personalized by choosing the correct targeting ligands such as proteins and antibodies which provide both selectivity and specific function. An understanding of the interaction at the atomic level between nanoparticles and proteins can provide insight into ideal modification strategies to maximize the potential of both nanoparticles and the antibody of choice for biomedical applications.
Analysis of the cellular protein interaction with theranostic nanotechnology provides a deeper understanding of the parameters and modification strategies to ensure the correct function is achieved. In the area of drug delivery, we investigated the functionalization strategies for the hybridization of organic nanoparticle drug carriers with inorganic imaging compatible nanoparticles, effect of size, and antibody bioconjugation on cell viability. The goal was to ensure the nanoparticle model minimized disruptions to the cellular structures while exacting its purpose for targeted localization or inducing a pharmacological effect.
For biosensor applications, we demonstrated a non-invasive alternative to glucose measurement via tear glucose with high selectivity and sensitivity through the conjugation of the lectin concanavalin A (Con A) with fluorescent nanoparticles. Through the Forster Resonance Energy Transfer mechanism, we were able to measure glucose levels as low as 0.03 mM with high selectivity and sensitivity to minute changes in glucose concentration. These findings provide a better understanding of merging antibodies/proteins with nanotechnology and their effect in a biomedical setting. Effective management of nanotechnology can potentiate a stronger physiological reaction, provide biomedical imaging relevance, and enhance biosensor development.
ENGINEERING LARGE GELATIN NANOSPHERES COATED WITH QUANTUM DOTS FOR TARGETED DELIVERY TO HUMAN OSTEOSARCOMA CELLS WITH ENHANCED CELLULAR INTERNALIZATION

Figure 2-1. Two-step desolvation method of gelatin nanosphere (GNs) synthesis. Stabilization of GNs was achieved by crosslinking with glutaraldehyde.
GNs were synthesized from Type A gelatin derived from porcine skin (175 Bloom, Sigma-Aldrich) using a modified two-step desolvation method (Figure 2-1) described by Coester et al. 1.25 g of gelatin was dissolved in 25 mL water under constant stirring and heating at 40°C. Once dissolved, 25 mL of acetone was added as a desolvating agent.
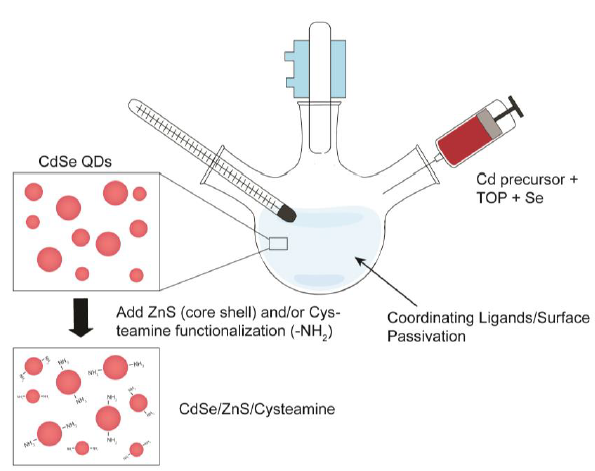
Figure 2-2. Synthesis scheme of CdSe/Zn/Cysteamine QDs.
Figure 2-2. Synthesis scheme of CdSe/Zn/Cysteamine QDs. Organometallic synthesis of the CdSe QD core was subject to ZnS capping to form a core shell entity. Amine (-NH2) functionalization to render the QDs water-soluble and biocompatible was achieved through a thiol-exchange reaction with cysteamine hydrochloride.
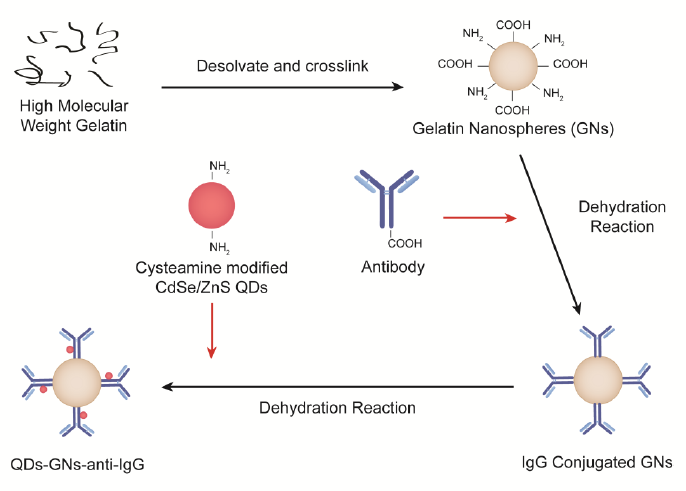
Figure 2-3. Functionalization scheme of Gelatin Nanospheres.
Figure 2-3. Functionalization scheme of Gelatin Nanospheres. Exploitation of the functional groups present on GN surface allows for the hybridization of QDs and bioconjugation of anti-IgG through a serious of dehydration reactions.
CELLULAR INTERACTION INFLUENCED BY SURFACE MODIFICATION STRATEGIES OF GELATIN-BASED NANOPARTICLES
Figure 3-3. Schematic of GNP modifications. Modification paths of 90 bloom-based GNP: Path A – Cadmium Selenium Cysteamine modified Quantum Dots (CdSe/ZnS/Cys QD) first; Path B – anti-human Immunoglobulin G Fab (anti-IgG) first. The order of modification exposes different groups on the surface for subsequent modifications. Modifications rely on the formation of amide (-CONH-) bonds to covalently stabilized the constituents. *constituents are listed in order of modification.
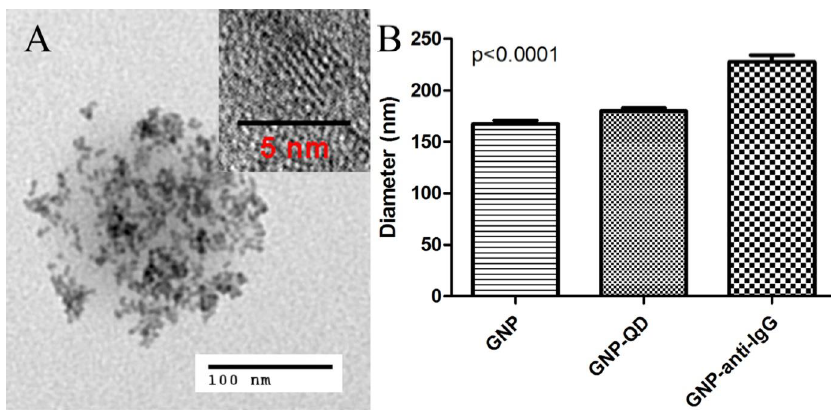
Figure 3-5. Microstructures of hybrid nanoparticles.
Figure 3-5. Microstructures of hybrid nanoparticles. A) TEM micrograph of hybrid nanoparticles GNP-QD is shown with clusters of hybridized QDs on the surface. B) Diameters of GNP indicate a significant increase with the bioconjugation of anti-IgG Fab from 167 ± 43 nm to 227 ± 50 nm (p < 0.0001). The same is observed when hybridized with CdSe/ZnS/Cys QD (180 ± 37 nm).
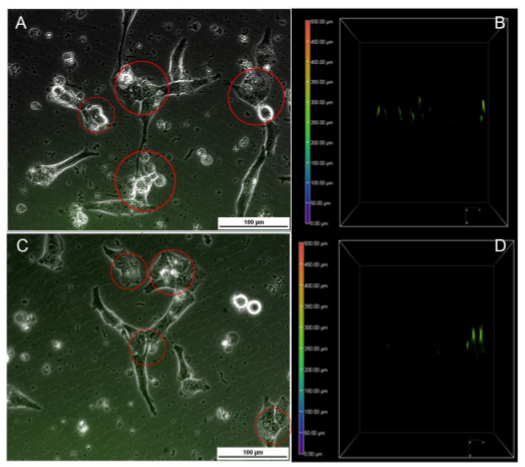
Figure 3-9. Cellular Interaction of GNP-anti-Ig/GQD and GNP-anti-IgG/QD/PEG with UTA-6 cells.
Figure 3-9. Cellular Interaction of GNP-anti-Ig/GQD and GNP-anti-IgG/QD/PEG with UTA-6 cells. Internalization and surface association is also observed in the fluorescent and brightfield overlay of A) GNP-anti-IgG/QD and B) GNP-anti-IgG/QD/PEG. Respective z-stacks are seen in B) and D). Taking 250 μm as the cell surface, all fluorescent signals detected below it were considered internalized. Nanoparticles are outlined in red.
BIMODAL IMAGING COMPATIBLE THERANOSTIC NANOPARTICLES FOR ANTI-ANGIOGENIC TREATMENT
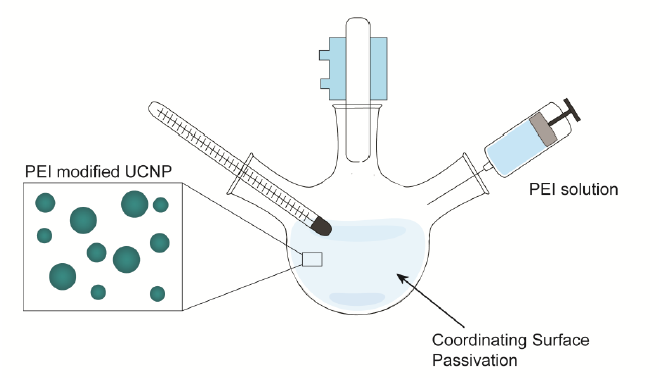
Figure 4-2. Synthesis schematic of upconversion nanoparticles modified with PEI. The synthesis occurs in a one pot reaction to grow the NaGdF4: Er, Yb UCNPs.
Water soluble Gd-based UCNPs doped with polyethylenimine (PEI), Er, and Yb were synthesized according to the method used by Longyi et al. (Figure 4-2). In short, 1.6 mmol gadolinium(III) nitrate hexahydrate (Gd(NO3)3·6H2O), 0.36 mmol ytterbium(III) nitrate pentahydrate (Yb(NO3)3·5H2O), 0.38 mmol erbium(III) nitrate pentahydrate (Er(NO3)3·5H2O), 8 mmol sodium fluoride (NaF), 0.7 g PEI (MW ~ 800 by LS, average Mn ~ 600 by GPC), and 30 mL of ethylene glycol; all purchased from Sigma-Aldrich, were combined in a one pot synthesis reaction to produce NaGdF4: Er, Yb (Gd-UCNPs).
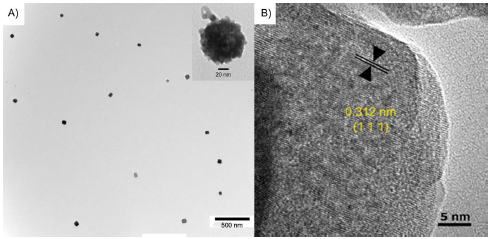
Figure 4-5. Upconversion antibody model characterization by TEM.
Figure 4-5. Upconversion antibody model characterization by TEM. A) Bare Gd-UCNP visualized by TEM. As shown in the inset, the anti-VEGF (outlined in red) was visualized on the Gd-antiVEGF UCNP. B) HRTEM shows the highly crystalline structure of the UCNP. The interplanar distance between adjacent lattice planes was 0.312 nm.
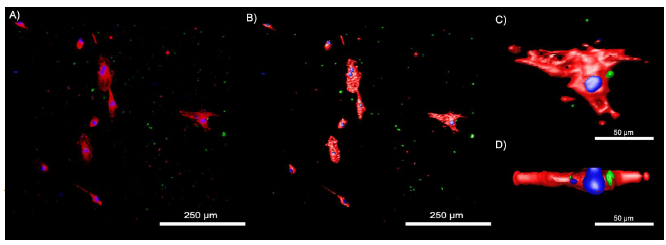
Figure 4-12. Confocal images of HUVECs treated with FITC tagged Gd-antiVEGF UCNPs.
Figure 4-12. Confocal images of HUVECs treated with FITC tagged Gd-antiVEGF UCNPs. A) Confocal images depicting the stained actin (red), cell nucleus (blue) and FITC tagged Gd-antiVEGF UCNPs (green). B) Corresponding isoform image. C) Magnified isoform showing the cellular interaction of Gd-antiVEGF UCNP. D) Z-stack of an endothelial cells demonstrating the intracellular location of the Gd-antiVEGF UCNP relative to the nucleus.
NANOSTRUCTURED BIOSENSOR FOR DETECTING GLUCOSE IN TEAR BY APPLYING FLUORESCENCE RESONANCE ENERGY TRANSFER QUENCHING MECHANISM

Figure 5-1. Illustration of the designed FRET transducer made of ConA-conjugating quantum dots (donor) and MG (acceptor) for detecting glucose.
Figure 5-1. Illustration of the designed FRET transducer made of ConA-conjugating quantum dots (donor) and MG (acceptor) for detecting glucose. Competitive affinity for glucose displaces MG to restore the quenched fluorescence. (b) Immobilization of the nanostructured FRET transducers on ZnO nanorod array deposited on silicone hydrogel.
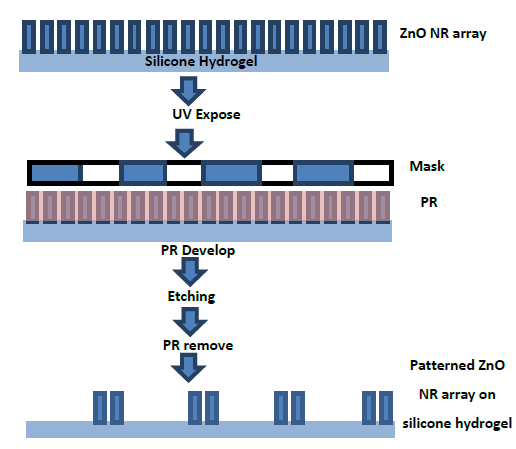
Figure 5-2. A photolithographic lift-off process for fabricating the patterned ZnO nanorod array on silicone hydrogel.
Silicone hydrogel with 150 μm in thickness was produced using a photochemical process. The patterned ZnO nanorod (NR) array was fabricated on the silicone hydrogel by a photolithographic lift-off process as visualized in Figure 5-2. Photoresist (PR; Shipley 1827) was deposited on the ZnO NR arrays by spin coating and exposed to UV (Karl Suss MJB3, Hg Arc Lamp) irradiation over a patterned photomask for 30 seconds followed with post-baking.
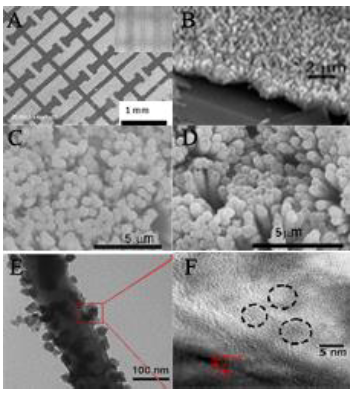
Figure 5-6. Characterization of nanomaterials by electron microscopy.
Figure 5-6. Characterization of nanomaterials by electron microscopy. A) SEM micrographs of patterned ZnO NRs deposited on a silicone hydrogel. The small inset is the photomask. B) The magnified SEM micrograph of ZnO nanorod array deposited on a silicone hydrogel. C) SEM micrograph of ZnO nanorods. D) SEM micrograph of QDs coated ZnO nanorods. E) TEM micrograph of QDs coated ZnO nanorods. F) HRTEM of CdSe/ZnS QDs.
CONCLUSION AND FUTURE DIRECTIONS
Theranostics is a constantly evolving field, providing new opportunities in developing new healthcare technologies at an affordable price.1 The application of nanoparticles is highly suitable for theranostics as it provides material-based properties, a framework for personalized medicine, and high-surface area to volume fitting for surface modifications. The goal of theranostics is to concurrently provide biomedically relevant diagnostic information and exert a pharmaceutical effect and overcome potential limitations of current technology. Applications of theranostic applications aim to enhance drug carriers, biosensors, and imaging entities.
For instance, gadolinium contrast agents have been proven to cause adverse effects if in its free ionic form rather than chelated.5,6 Through transmetallation, the Gd ions can be replaced in the chelator by Fe, Ca, Zn, and Cu ions, where thereby the Gd may freely interfere with cation channels leading to splenic degeneration, liver necrosis, 5 etc.5Cases of neurotoxicity have also been reported.
Drug delivery can be affected by natural barriers such as the hepatic first pass, the blood brain barrier, and diffusion through fenestrations causing a loss of therapeutic concentration and therefore shortening the duration of treatment or not achieving the therapeutic threshold to exert a therapeutic response.
The application of nanoparticles for theranostics is dependent its functionalization with targeting ligands, proteins, and antibodies for specificity and functionality. It is already understood nanoparticle possess far superior properties that can address the shortcomings of current biomedical technologies. However, as a whole, nanoparticles and its functionalization is not yet understood with regards to functionalization strategies and cellular interactions.
Source: The University of Western Ontario
Author: Wai Hei Tse
>> IoT Projects in Medical Field for Final Year Students
